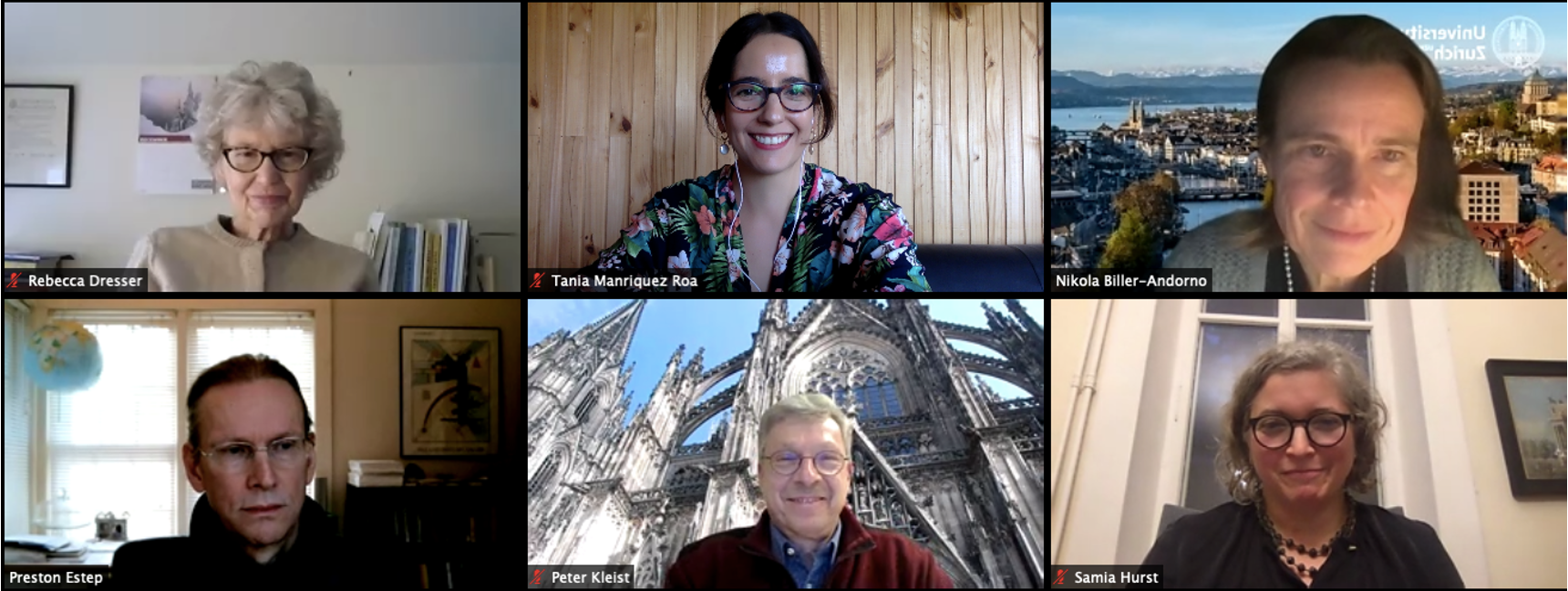
Webinar on Self-Experimentation in Times of Covid-19
In the early stages of the COVID-19 pandemic, several scientists conducted experiments on themselves voluntarily and deliberately with the goal of finding a vaccine against the virus. This practice is not new. In the past, scientists and physicians embarked on self-experimentation to develop polio, typhoid, and rabies vaccines. Famous self-experimentation research includes Barry Marshall’s ingestion of helicobacter culture to prove that the bacteria cause gastrointestinal disease and Werner Forssmann’s insertion of a catheter into his own heart to demonstrate this procedure could be done safely. Although self-experimentation has helped to elucidate the aetiology of treatable diseases and to advance in medical procedures, some self-experiments led their subjects to permanent disability and death.
Self-experimentation was a common practice in medical research before the institutionalisation of ethical research through the Nuremberg Code, the Helsinki Declaration, institutional review boards and research regulations. Until the late 20th century, researchers viewed self-experimentation as an ethical approach to doing science, given the ethical implications of exposing others to the potential negative effects of untested interventions. Today, this idea is far from mainstream research ethics. However, based on the premise that exceptional times demand exceptional actions, the urgency to find and develop a vaccine for COVID-19 has fuelled a renewed debate on the ethics of self-experimentation.
In this webinar international experts who hold different views on self-experimentation discuss the potentials and risks of this research practice.
Speakers:
- Preston Estep, Founder, Rapid Deployment Vaccine Collaborative ‒ RaDVac
- Rebecca Dresser, Daniel Noyes Kirby Professor, Washington University in St. Louis
- Peter Kleist, Director, Zurich Cantonal Ethics Committee
- Samia Hurst-Majno, Director of the Institute for Ethics, History and Humanities, University of Geneva
Hosts: Nikola Biller-Andorno and Tania Manríquez Roa, University of Zurich
Organising institutions: University of Zurich (IBME) and Swiss Medical Weekly
Organisers: Tania Manríquez Roa, Nikola Biller-Andorno, and Dominik Bolliger
Read the viewpoint «Going first: the ethics of vaccine self-experimentation in coronavirus times» , by Tania Manríquez Roa and Nikola Biller-Andorno
Read the case report «Self-experimentation in the development of COVID-19 vaccines and the release of product formulas» by Tania Manríquez Roa and Nikola Biller-Andorno (forthcoming) In Oxford Casebook on Ethical Issues in Epidemic Health Research (Bull, S. and Parker, M. Eds.) Oxford: Oxford University Press.
Read the webinar report «Altruism, recklessness, or something else? A summary of the forum on self-experimentation in the time of COVID-19» by Sophie Gloeckler and Tania Manríquez Roa.
Here you can watch clips of this webinar.